- What Is Avogadro’s Number
Avogadro’s number is defined as the number of particles (atoms, ions, molecules and formula units) in one mole of a substance is called Avogadro’s number. The particles may be atoms, ions, molecules or formula units. It is represented by NA. It is a constant whose value is 6.02×1023.
- What Is Avogadro's Number Based On
Avogadro's number is based on the principle that at same pressure and temperature equal volume of gases contain equal number of molecules.
- Does Avogadro’s Number Have Unit
Avogadro's number have unit. It has unit mol-1.


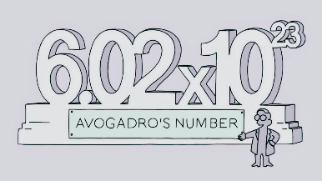
0 Comments